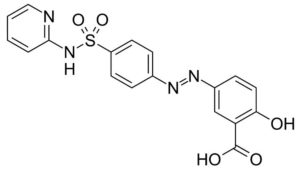
AZELAIC ACID & HAIR GROWTH
Elzbieta E.Brand-Garnys M.Sc., Dr.Hans M.Brand
Hair grows everywhere on our body except on the palms of our hands and the soles of our feet. An average human adult is the proud owner of approximately 150,000 hairs on her/his head, but also loses up to 100 of them per day. A lot of people, however, own a rather broad hair line, that even may spread over the complete skull. Take William: just a bit of downy hair on the back of his head. He claims where hair grows there is no room for brains. It is quite remarkable indeed that the vast majority of our dear politicians have a sumptuous full, thick head of hair, frequently unkempt.
VARIOUS FORMS OF HAIR LOSS.
Contrary to William many individuals really suffer from hair loss (alopecia), especially females. One of the conditions leading to thinning hair is known as hereditary hypotrichosis simplex. The responsible gene, APCDD1, has been identified and causes hair follicles to shrink (Christiano2, 1998). Victims of genetic hairlessness also frequently face the absence of eyelashes and eyebrows. There are sincere indications that the number of people condemned to genetic baldness is limited to less than a hundred individuals worldwide; scant comfort for those with an unwanted naked scull.
Virtually all individuals will face sooner or later involutional alopecia (old age hair loss) and that can only be delayed by providing the hair follicles with a frequent shot of essential nutrients (B vitamins and some metal ions). A low-protein diet or severely calorie-restricted diet frequently causes temporary hair loss. Alopecia areata (spot baldness) and alopecia universalis (total body hair fall-out, including eyebrows & eye lashes) are auto-immune disorders for which no general treatment is available.
With Alopecia areata hair loss is observed resulting in smooth, round patches about the size of a coin or larger. It can, rarely, result in complete loss of scalp and body hair. This disease may affect children or adults of any age. The cause of alopecia areata is unknown, having said that the affected victims are generally in excellent health. In most cases, the hair regrows without intervention. Alopecia areata may also be observed with pregnant woman: during pregnancy the hair will be growing with increasing intensity (pregnant woman frequently have beautiful hair) but after delivery many hairs swiftly enter a dormant stage. Within two to three months, some women will notice large amounts of hair coming out in their brushes and combs. This can last up to half a year, but resolves completely in most cases. In a number of cases sulfasalazine or anthralin (1,8-dihydroxyanthron) offer an effective treatment for alopecia areata, but superior results are obtained using azelaic acid, without the unwanted side effects of sulfasalazine and/or anthralin7 (1,8-dihydroxyanthron).
SULFOSALAZINE
Male pattern hair loss (MPHL; androgenic alopecia) develops under the influence of androgenic hormones and is probably the most important reason for the development of baldness. Androgenic alopecia is not life-threatening. The research investments in hair growth solutions are quite significant indeed, and comparable to the investments made in research on diabetes, cancer and cardio-vascular conditions. Investments in hair growth research are justified as the hair follicles are an important scientific model for understanding important aspects of human cell biology, organ system developmental biology, immune response medicine, the process of controlled cell regeneration & differentiation, stem cell technology and human genetics (ref. the American Hair Loss Association1). Apart from the scientific value of studying hair growth there is also a significant commercial interest, especially in the Asian Pacific Region, Japan & China.
Abnormal levels of androgenic hormones are responsible for the majority of cases of hair loss. Other frequently occurring reasons are the use of medicinal products (chemotherapeutics, blood thinning agents, beta-blockers and oral contraceptives). Particular fungal infections (ringworm) caused by Trichophyton rubrum and related organisms may result in hair loss.
THE CYCLE OF HAIR GROWTH.
Hair is made up of keratin, a protein that is made in the hair follicle. Hair grows out of the follicle that is located in the living dermis and reaches skin surface through the epidermis. The hair grows through the shaft of the follicle and becomes visible above the scalp. The hair follicle has the shape of a funnel with the narrowest part on the outer surface of the epidermis, and extends down into the living dermis. At the base of the follicle the papilla ifs found, which contains the capillaries (tiny blood vessels that nourish the living cells). The living part of the hair is the very bottom part surrounding the papilla, called the bulb; the remainder of the hair is gradually pushed up to the outside world and is truly dead material. The bulb cells swiftly divide (1-3 days), depending on the location on the skin, but markedly much faster than any other cell in the human body.
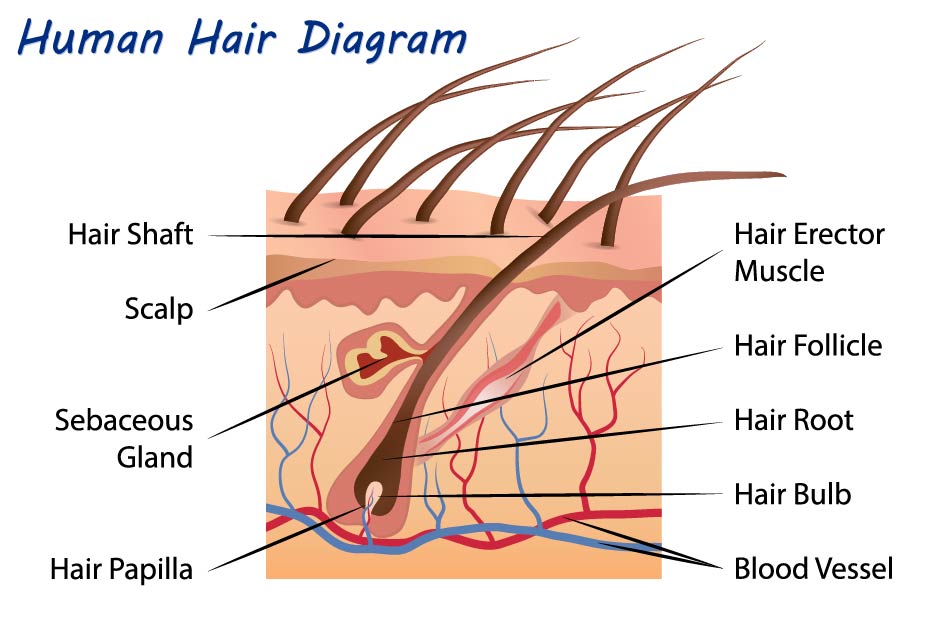
Hairs grow in three stages:
- The anagen stage: this is known as the growth stage of the hair. Growth starts in the papilla. Hairs are a number of years in the anagen stage. The majority of hairs is in the anagen stage.
- The catagen stage: follows the anagen stage. During the catagen stage the hair follicles regain their strength; the melanin formation comes to a full stop because of apoptosis of the follicular melanocytes. The catagen stage lasts for approximately 2-3 weeks. The blood supply to the papilla comes to a full stop, and consequently the hair is not nourished anymore.
- The telogen stage: follows the catagen stage and is also named the resting stage. The follicle remains dormant for 2-6 months. After this period the follicle starts to grow again and a new hair shaft will be formed. The old hair is detached and shed off, and the anagen stage is entered again.
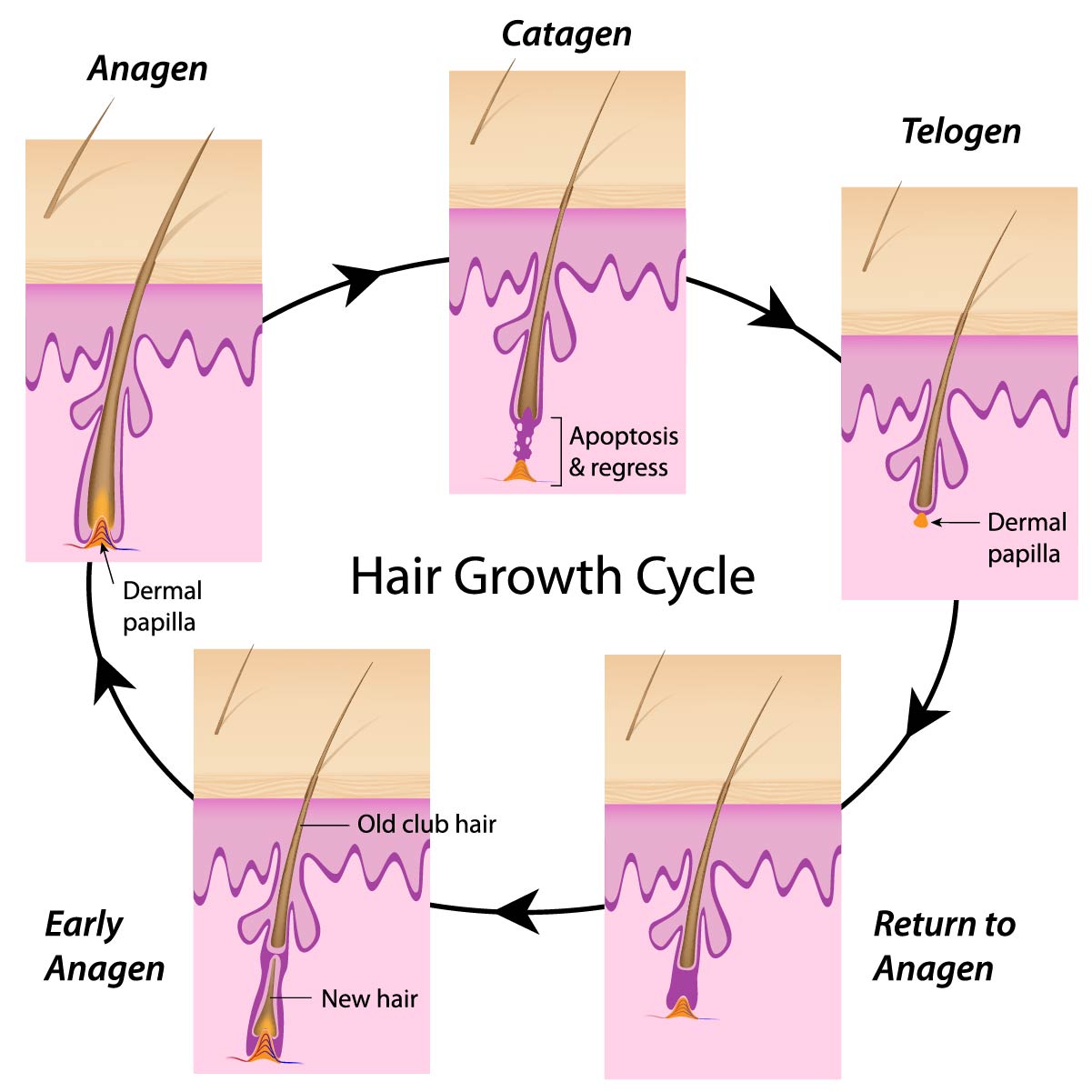
The majority of hairs are in the anagen stage (80-90%). Only about 1% of hairs are in the catagen stage while 10-15% hairs are in the telogen stage.
In practice there is a fragile equilibrium between the anagen, catagen and telogen stage. This equilibrium can be manipulated, such as what has been done with the angora mouse mutant. Angora mice have abnormally long hair, and this is due to an increased time span where the follicles remain in the anagen stage of the cycle. So, the hair keeps growing and growing (Pennycuik3, Sundberg4).
This condition is due to mutations in the gene that is responsible for the production of the fibroblast growth factor 5 (FGF-5; Hébert5). These mutations are responsible for greatly reduced FGF-5 production. FGF-5 appears to be needed for the progression of the hair cycle from the anagen stage to the catagen stage. Without FGF-5, the timing is delayed with the obvious results. Eventually, the catagen stage is reached anyway; the hair matrix cells have only a limited capacity for cell division (the Hayflick factor). It has also been proposed that another growth factor may substitute FGF-5, but with lower efficacy. However, there is little doubt that FGF-5 is determining hair growth.
MORPHOLOGY OF HAIR.
Morphologically there are three types of hair. Vellus hairs are short and fine, soft and usually not pigmented. Terminal hairs are large and intensively pigmented (90% of the hairs on the chest, trunk, shoulders, legs, and arms of men are terminal hairs; woman have only ~4500 terminal hairs in the same regions). Intermediate hairs occur on the scalp, and they demonstrate a morphology between terminal and vellus hairs. Intermediate hairs contain only a moderate amount of pigment, less than found in terminal hairs.
Each type of hair undergoes repeated cycles of active growth and rest; the relative duration of each cycle varies with the age of the individual and the region of the body where the hair grows. The length of the cycle is often modified by a variety of physiologic and pathologic factors. The balding process is a conversion of the follicles so that they produce vellus hairs rather than terminal hairs.
ANDROGENIC ALOPECIA.
The hair follicle contains stem cells, dispersed in the basal layer of the outer root sheath and in an area called the bulge. From this reservoir stem cells migrate to the hair matrix and start to divide and differentiate. Their behavior is largely controlled by cytokines (signaling proteins that enable cells to communicate) that are produced by cells of the dermal papilla. Dermal papilla cells, and some cells of the inner and outer sheaths of the follicle, have androgen receptors in their cytoplasm and nucleus, and are androgen dependent. Androgens indirectly control hair growth by influencing the synthesis and release of cytokines from the dermal papilla cells.
Androgens are steroid hormones that stimulate or control the development and maintenance of male characteristics by binding to androgen receptors. The primary and most well-known androgens are testosterone, dihydrotestosterone (DHT) and androstenedione.
Androgens bind to their receptors both in the cytoplasm and the nuclei of dermal papilla cells and some cells of the sheaths of the follicle, but only if the hair is in the anagen or telogen stage. Upon the formation of the complex of the androgen and the receptor cytokines are produced that are essential for hair growth. When the formation of the complex is inhibited or made impossible, cytokine production will also be inhibited and thus hair growth is put to a full stop. Retinoic acid (vitamin A), if used for a long time, may reduce the number of active androgen receptors by 30-40 %, while pyridoxine (vitamin B6) reduces cytokine production by 40-50%.
TESTOSTERONE DIHYDROTESTOSTERONE
The most dramatic influence on dermal papilla cells is induced by dihydrotestosterone, and this the major cause for hair loss. It is produced in an equilibrium reaction from testosterone, catalysed by the enzyme 5-a-reductase. Sportsman using testosterone supplements to increase their muscle volume will automatically also increase the concentration dihydrotestosterone, and that results in baldness. Testosterone supplements are strictly forbidden in organised sport (WADA).
There are two forms of 5-α-reductase. Type 1 (259 amino acids) resides mainly in sebocytes but also in epidermal and follicular keratinocytes, dermal papilla cells and sweat glands. Sebocytes are highly specialized, sebum-producing epithelial cells that release their content by rupture of the cell membrane and cellular degradation (holocrine secretion). Type 2 (254 amino acids) is located mainly in the epididymis, seminal vesicles, prostate and fetal genital skin as well as in the inner root sheath of the hair follicle. In particular substrates that selectively bind to type 1 5-a-reductase may be considered for the treatment of androgenetic alopecia.
Inhibition of 5-a-reductase type 1 is therefore an answer to androgenic alopecia. This may be done using pharmaceutically active products such as dutasteride, finasteride or Minoxidil®; a major consideration is that these products (all three are alpha blockers) only enable to produce vellus hair and to some extent intermediate hairs. These products are not allowed in cosmetic preparations because of the possible very sincere side effects. These side effects originate from the fact that these products are also used to treat benign prostatic hyperplasia (BPH) in men with an enlarged prostate.

SAW PALMETTO (SERENOA REPENS)
There is only a limited number of cosmetically allowed 5-a-reductase inhibitors available. Saw palmetto, alfalfa, Japanese pagoda tree, red clover and the often praised Indian mulberry (noni fruit) have been reported to exhibit 5-a-reductase inhibition properties. It has been suggested that the aromatase activity10 is responsible for these effects8,9. The net effect of these botanicals is, however, limited and not at all comparable to azelaic acid.


NONI FRUIT (MORINDA CITRIFOLIA)
AZELAIC ACID
Azelaic acid is a very potent 5-a-reductase inhibitor (type 1). According to Stamatiadis11 5-a-reductase inhibition is already detectable at an azelaic acid concentration as low as 0,2 mMol/l. Inhibition is complete at a concentration of 3 mMol/l, equivalent to ~0,6 mg/l. Stamatiadis also studied the inhibitory effects of zinc sulphate (3-9 mMol/l) using an in vitro assay with 1,2[3H]-testosterone as substrate; also zinc sulphate showed to be a potent 5-a-reductase inhibitor. An additive effect of these two inhibitors was observed. Pyridoxine (vitamin B6) potentiated the inhibitory effect of zinc sulphate, but not of azelaic acid. This observation suggests that different mechanisms are involved. Simultaneous use of the three products showed to be already effective for the treatment of androgenic alopecia, indicative for a powerful synergy.
Azelaic acid is poorly soluble in water, but relatively easy soluble in glycols, preferably 1,3-propanediol (INCI Propanediol), 1,3-butanediol (INCI Butylene Glycol) and 1,2-pentanediol (INCI: Pentylene Glycol) and Diethylene glycol monoethyl ether (INCI: Ethoxydiglycol), and mixtures thereof. Another interesting vehicle for dissolution of azelaic acid is composed of polysorbate 85 (PEG-20 sorbitan trioleate) and poloxamer 10114,15. The micro-emulsion obtained is a superb carrier for azelaic acid, with a good degree of bioavailability. The bioavailability can be further improved using phosphatidylcholine dissolved in a suitable solvent such as isopropyl palmitate or ethylhexyl stearate. To the phosphatidylcholine solution a cold (4°C) aqueous/glycerol (1:1) solution of poloxamer 407 containing azelaic acid is added and homogenized using high shear. The obtained organogel, according to Scartazzini16, has a very high degree of bioavailability, enabling to significantly reduce the concentration azelaic acid while guaranteeing the full functionality of azelaic acid.
The powerful combination {azelaic acid + zinc sulphate + vitamin B6} for the treatment of androgenic alopecia is cosmetically suitable contrary to the steroidal and non-steroidal pharmaceutical preparations. In addition, azelaic acid has a superior toxicological profile. Side effects of azelaic acid boil down to the particular cosmetic properties: skin lightening at the site of application, a slight risk of hypertrichosis, and [seldom] slight skin irritation.
Combinations of minoxidil and azelaic acid are commercially available, despite the unwanted side effects of minoxidil. Both products work on the basis of different mechanisms of action in preventing baldness. The combination of the two would work more effectively than either alone. Commercial products contain up to 15% of high purity azelaic acid and 5% minoxidil. These high concentrations are explained because of the poor bioavailability of especially minoxidil. Lower but equally effective preparations can be made using transdermal preparations based on phosphatidylcholine-based organogels12,13.
Literature cited.
- http://www.americanhairloss.org/hair_loss_research/introduction.asp.
- W.Ahmad, M.F.Haque, V.Brancolini, H.C.Tsou, S.Haque, H.M.Lam,V.M.Aita, J.Owen, M.deBlaquiere, J.A.Frank, P.B.Cserhalmi-Friedman, A.Leask, J.McGrath, M.Peacocke, M.Ahmad, J.Ott, A.M.Christiano, Alopecia Universalis Associated with a Mutation in the Human hairless Gene, Science 279,720,(1998).
- P.R.Pennycuik, K.A.Raphael, the angora locus go) in the mouse: hair morphology, duration of growth cycle and site of action. Genet.Res. 44,283,(1984).
- J.P.Sundberg, H.Peters, Cyclic alopecia in Msx2 mutants: defects in hair cycling and hair shaft differentiation, Development 130,379,(2003).
- Jean M. Hébert, Opens overlay Thomas Rosenquist, Opens overlay Jürgen Götz ★, Opens overlay Gail R. Martin Jean M. Hébert, Opens overlay Thomas Rosenquist, Opens overlay Jürgen Götz ★, Opens overlay Gail R. MartinJ.M.Hébert, T.Rosenquist, J.Götz, G.R.Martin, FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutationsFGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations, Cell 78,1017,(1994).
- C.A.Higgins, L.Petukhova, S.Harel, Y.Y.Ho, E.Drill, L.Shapiro, M.Wajid, A.M.Christiano, FGF5 is a crucial regulator of hair length in humans. Proc.Nat. Acad.Sci., 111,10648,(2014).
- S.Sasmaz, O.Arican, Comparison of azelaic acid and anthralin for the therapy of patchy alopecia areata: a pilot study. Am.J.Clin.Dermatol., 6,403,(2005).
- E.R.Simpson, M.S.Mahendroo, G.D.Means, M.W.Kilgore, M.M.Hinshelwood, S.Graham-Lorence, B.Amarneh, Y.Ito, C.R.Fisher, M.D.Michael, C.R.Mendelson, S.E.Bulun, Endocr.Rev., 15,342,(1994)
- E.R.Simpson, Y.Zhao, V.R.Agarwal, M.D.Michael, S.E.Bulun, M.M.Hinshelwood, S.Graham-Lorence, T.Sun, C.R.Fisher, K.Qin, C.R.Mendelson. Recent Prog. Horm.Res. 52,185,(1997).
- M.J.Balunasa, R.W.Brüggemeier, A.D.Kinghorn, Natural Products as Aromatase Inhibitors, Anticancer Agents Med.Chem., 8,646,(2008).
- D.Stamatiadis, M.C.Bulteau-Portois, I.Mowszowicz, Inhibition of 5α-reductase activity in human skin by zinc and azelaic acid. Brit.J.Derm., 119,627,1988.
- S.Murdan. A review of pluronic lecithin organogel as a topical and transdermal drug delivery system. Hospital Pharmacist 12,267,(2005).
- R.Kumar, O.M.Katare. Lecithin Organogels as a Potential Phospholipid-Structured System for Topical Drug Delivery: A Review. AAPS Pharm.Sci.Tech., 2005; 6 (2) Article 40.
- S.Kalepu, M.Manthina, V.Padavala. Oral lipid-based drug delivery systems -an overview. Acta Pharm.Sinica 3,361,(2013).
- S.Raut, S.Singh Bhadoriya, V.Uplanchiwar, V.Mishra, A.Gahane, S.Kumar Jain. Lecithin Organogel: a unique micellar system for the delivery of bioactive agents in the treatment of skin ageing. Acta Pharm.Sinica 2,8,(2012).
- R.Scartazzini, P.L.Luisi, Organogels from Lecithins. J.Phys.Chem., 92,829,(1988).
